STABILITY 🔀⏩️LIFESPAN
The lifespan of objects in the universe is dependent on the stability of its unit ©2023 akshay Sanghavi
All matter moves towards stability.
Atom is more stable when it is in a lower energy state and so it tries to move towards the lesser energy state.
Unstable atom isotopes will spontaneously decay and change to another more stable element. The rate of radioactive decay is inversely proportional to the stability of the isotope. Radon-222 has a half life of just 4 days so every 4 days it will reduce to half. Which creates the spontaneous move towards a more stable form. Universal law of survival: a stable atom can exist for billions of years. Xenon-124’s half life is 18 billion trillion years. Despite its incredible lifespan and stability, it’s not immortal: it will also eventually die and turn to tellurium-124.
I have been thinking about the different lifespans of biological species on our planet. My fascination and desperate curiosity to unravel the mystery of variable lifespans has obsessed me with constantly researching the potential causes. I have been sharing my findings in some of my previous posts like Mechanism of Aging, Headwaters, Autologous Regulation and Agents of Time. In the last few posts I have shared the realization that a cell’s destiny is strongly linked to regulatory changes enforced by regulators like non coding RNAs. Of course, there is incredible complexity to this process. Nothing else on this planet has brilliant, complex, autonomous engineering like our biology. But can there be one simple factor behind the variance that we see in the lifespans? It’s not size for example as the exceptions demonstrate. My mind is blown by the study by Professor Richard Dixon on Ginkgo Biloba tree. So much that it features in all my longevity related posts since it was published. If these hardcoded transcription plans are making deliberate changes starting after puberty setting off a cascade that ends in death, then how did Ginkgo Biloba make adaptive changes to bypass such a universal recycling mechanism of Nature? Professor Dixon called it almost immortal. The best part is that not only is it almost immortal but it’s almost immortal in a youthful state! Who wants to live a thousand years looking like shrunken bag of bones and crumpled skin? So, what is this one single cause of lifespan variance? We will arrive at that after some more deliberation.
As we read in a paper by Morimoto and Labbadia called ‘Repression of the heat shock response is a programmed event at the onset of reproduction’ that just after puberty there is a change that reduces the ability of chaperones, that are key to the protein production process in our cells, by 60%-70%. This results in higher number of malformed proteins. This starts a cascade of many other drops in important functions in the cell and these drops snowball making us age. For example, as we read in Agents of Time, the debris of the 450 billion cells that die every single day in our body is cleared by phagocytes. But thanks to various regulatory changes and their ensuing cascades their mitochondrial batteries begin to fail them. This leads to accumulating number of sharp debris that enters cells and causes 1 quadrillion double strand DNA breaks everyday! In this chain of events germline cells triggered epigenetic changes that resulted in the fall of efficiency of protein production chaperones. What if these epigenetic changes were not allowed to be made or after they were made they were ‘repaired’ back to their original epigenetic configuration. In order to prioritize reproduction the germline stem cells trigger epigenetic repression of heat shock proteins which act as chaperones to support protein production and as stress response. When we get a hurt while playing sports the injury is repaired over a period of time. What if similarly the epigenetic mark that germline stem cells make after puberty is demethylated or removed to restore back the full strength of heat shock proteins? That would stop the chain of events that eventually cause so many double strand DNA breaks every day. Just to demonstrate how this cascade snowballs when those breaks keep happening in the DNA, even though almost all are repaired, they succeed in creating roadblocks in the hurtling train of transcription in our DNA. This than has serious repercussions as many transcription programs get blunted. In the Agents of Time blog another change causing aging is mentioned wherein our long, coding and noncoding, transcripts begin to fade. The authors of the cited paper also mentioned that these longest transcripts were activating prolongevity genes. Thereby repressing or silencing the genes associated with making us live longer. So when we prevent or significantly reduce the huge number of double strand DNA breaks we restore smooth transcription and thereby prevent the loss of longer transcripts and silencing of longevity genes. Do you see where this is leading us?
1,400 year old Gingko Biloba Tree
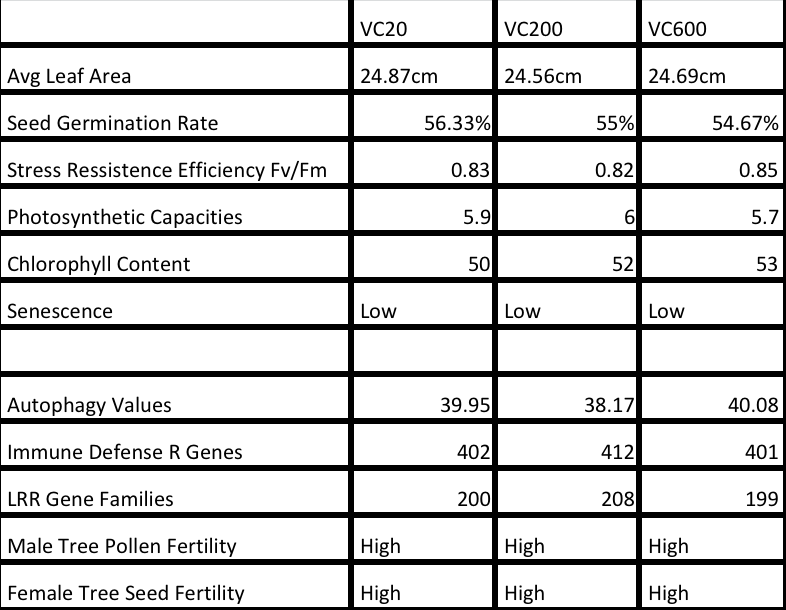
The mechanism that Ginkgo Biloba tree has figured out is probably how to keep its epigenome stable! We are a collection of cells and all cells have the same DNA and yet they are transformed into 200 types to form eye and stomach and liver and skin etc. The DNA is the same but each type of cell has its own configuration of epigenetic marks on its DNA. These marks do not change the DNA physically but result in silencing majority of the genes and activating only the10%- 20% that transform it into its type of cell. Around puberty we achieve our homeostatic peak where all our systems are working at their best. But due to the changes mentioned above we experience what is called as ‘epigenetic’ drift where the beautiful epigenetic configuration found around puberty is slowly changing leading to unwanted genes being activated and wanted genes becoming silent. This epigenetic drift manifests into what we know as aging. The Ginkgo Biloba tree does not seem to succumb to this epigenetic drift which can be seen in almost all multicellular life forms leading to cell nuclear instability that slowly grows into cellular, tissue and organ instability. In a fascinating paper titled ‘Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees’ by Professor Richard Dixon and Dr. Jinxing Lin et.al. the authors try to reveal the longevity mechanisms of Ginkgo Biloba trees. The table below shows the miraculous stability of really old Ginkgo Biloba trees:
The authors selected nine trees for further study and divided them into three groups: 20 y (15Y, 20Y, and 22Y, young trees; VC20), 200 y (193Y, 211Y, and 236Y, older trees; VC200), and 600 y (538Y, 553Y, and 667Y, oldest trees; VC600). Below we can see the comparison of the average leaf area, seed germination rates, efficiency of stress resistance, photosynthetic capacity and chlorophyll content of the 3 groups:
Unlike almost all plants and trees that grow old and die Ginkgo Biloba’s environmental stress resistance, photosynthesis capacity, autophagy, sexual fertility and immune defense doesn’t drop with age! It’s cambial cells equivalent of our stem cells remain active even in oldest trees in the group but tempered with reduction in cell division, expansion and differentiation allows it to remain young without any uncontrolled growth or senescence. In human aging we begin to lose stability of the epigenome and our transcription track and its machinery leading to loss of beneficial genes and activation of unwanted genes. The exact opposite is happening with Ginkgo Biloba tree. In the same paper authors compared around 27,500 genes of the three groups: 20 year old, 200 year olds and 600 year olds there was only 4.4% difference in gene expression between the young trees and the older trees! How does Ginkgo Biloba tree maintain such stability of its epigenome, transcription, gene and protein expression???
This kind of beneficial stability is quite different then the longer lived species: the latter like Naked Mole Rats or Bowhead whales have some protective genes that are overexpressed even in old age. That does give longer than average life span associated with their species but they do grow old and die. Whereas Gingko Biloba trees seem to live forever in youthful prime.
As I had shared in my post Mechanism of Aging, Nature manages optimum levels of various activities and functions in our biology by the triage of activators and inhibitors and sensors. This works like a tap of hot water and cold water and a thermometer. Equilibrium is when taps are turned just the right amount to create ideal temperature of water. Any disturbance in this ratio can cause either too hot water or too cold water causing damage. In our prime, just after puberty, we enjoy optimum equilibrium or homeostasis between the activators, inhibitors and sensors. But soon after starts the slow offsets in this balance leading to all the accumulation of unrepaired damage and nuclear instability which manifests as age related changes. We can see and feel this happening especially after our 50s but since it’s not overnight and since we see others too showing similar negative changes we accept them. Ginkgo Biloba has been around longer than us: for more than 270 million years. Whereas we humans have been around only 200,000 to 300,000 years. Ginkgo Biloba has had 270 million more years to win the adaptation lottery. We humans too have won another genetic lottery: of higher intelligence that is compounding rapidly like an umbrella curve. We are developing great technologies and tools like CRISPR and AI and will soon figure out how to safely inculcate stability in our epigenome and nucleus. Then we too will have the option to live for thousands of years in the prime of our youth. Every known thing in observable universe seems to be recycled. The stars too die and leave behind a super dense spinning ball with no fusion or light just fading heat. These white dwarfs or neutron/pulsar remnants and planets and their debris all get recycled by black holes. All of the galaxies are probably held together by the gravitational pull of the super massive black hole at their core. This central black hole eventually will eat up the entire galaxy and break it down into fresh units of matter and radiation and spew them out as astrophysical jets long distances into space. So all the different bodies in a galaxy are broken down to sub atomic particles in the intense gravitational crush of the super massive black hole and slowly recycled into basic units of matter and radiation which then forms new stars and planets. So to outwit such a pervasive norm of recycling in the universe is a phenomenal achievement of Ginkgo Biloba.
Super massive black hole spewing recycled matter and radiation deep into space. By Newsweek.
Has Ginkgo Biloba developed some technology based in the futuristic world of science fiction? No. We see some mindblowing processes in biology all around us. During early embryogenesis there is global epigenetic reprogramming. For example in male embryos 96% of the methylation is wiped out to clear all accumulated errors from parents and later globally re-methylated as per template to create a brand new error free baby. This is the reason why Nature has created a program of aging so that continuous recycling will ensure cleaning out of errors every generation. So such an all encompassing and powerful epigenetic reprogramming technology is already being used in every embryo. Second technology is of self repair. Everyone of us would have got various degrees of hurt as a child while playing and see how over a few days or weeks it’s fully healed. Third technology is seen in some reptiles that regrow limbs even after they are fully severed. So biology already has some incredible engineering technologies to reset and restore any disturbance back to its optimum configuration. Can these technologies be harnessed to restore any erosion in our epigenome so that after every disturbance, whether it is stochastic or programmed, the epigenome is reset back to its optimum, youthful pattern?
To understand the cause of nuclear and cellular instability further let us borrow from my last post Agents of Time: We read that Aging is associated with loss of longer transcripts including long non coding RNA: https://www.nature.com/articles/s43587-022-00317-6
Plus they found: ‘we find that in humans and mice the genes with the longest transcripts enrich for genes reported to extend lifespan, whereas those with the shortest transcripts enrich for genes reported to shorten lifespan’
This is one of main reasons why longer transcripts fade away with aging:
https://www.nature.com/articles/s41588-022-01279-6
‘The drops were not due to drop in promoter activity or drop in RNAP II activity. Even the launch of transcription was unaffected. The authors postulate that DNA breaks interrupted and stalled transcription – and as longer genes/transcripts have greater chances of being broken somewhere they can be the primary cause of their reduced transcription during aging.’
A recent paper gives us glimpse of one of the ways this loss of long non coding RNAs cause the damage to tissues and organs during aging:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10834948/
“LncREST localises to chromatin (the structure in which DNA is organised in the cell). Its main function is to facilitate the localisation of key proteins in the process of DNA replication and DNA damage repair where they are needed. In fact, the absence of lncREST has been shown to cause impaired stress signalling, leading to the accumulation of severe DNA defects and, ultimately, cell death“
A few deliberate changes like the sudden massive drop in heat shock chaperone efficiency just after puberty start this cascade of errors leading to the instability of epigenome, genome, nuclear architecture and the cell. If these errors can be stopped/repaired as soon as they occur we too can’ live forever’ in youth like the Ginkgo Biloba tree. E5 does this partially by feeding older cells and its nucleus suffering from epigenetic drift with regulatory workers like longer transcripts from a youthful system that fill in some of the gaps or missing pieces in the old cell/nucleus. This restores the broken epigenetic pattern partially back to its original template. The amazing part is that old, damaged cells bounce back to youthful healthy fitness once it’s epigenetic pattern is reversed closer to its optimum design. This is a safer engineering intervention than any that needs the cell to move towards pluripotent state. As it creates a fluid epigenetic state which can lead to tripping into loss of cell identity and loss of controls which can result in development of cancerous growth. The idea is to create stability in the epigenome not fluidity.
We already have the engineering peak around puberty where our biology works beautifully. But there are a thousand negative changes that occur as we age. We have to now learn how not to slip into the epigenetic drift and to keep our cell stable at its homeostatic peak as long as we want. Ultimately, we want to install a Ginkgo Biloba type of mechanism that restores the cell and its nuclear architecture after any disturbance. The secret to long youthful lifespan is achieving long lasting nuclear stability after reaching adult biology. We just have to observe the Universe to unmask this secret. Stability is the difference between a lifespan of 4 days and 18 billion trillion years.


28 comments:
Excellent! The question is why some species atoms are at a lower state and live longer? What could determine the program of maximum lifespan?
Hi well I have tried to answer that in the post. They have learnt to maintain stability of their nuclear architecture-for example of their epigenetic landscape. Which ensures that there is very little change in their gene expression since they reach young adulthood. Maintaining that for thousands of years is truly remarkable. I have added a table of some key functions of Ginkgo Biloba tree: a 600 year old tree is as young as a 20 year old tree!
Cool, thank you. Do you think some lipid supplements, like pentadecanoic acid, phosphatidylcholine and cell membrane strengthening supplements can maintain integrity and stability of the architecture better?
Hi those supplements would be useful as with aging we see a loss of the impermeability of cell membranes. These supplements could probably delay such loss. But they may not be able to fortify enough to stop cell debris from penetrating the nucleus and causing double strand DNA breaks. In fact we suffer on average 1 quadrillion of such cuts every day! We have to admire our biology as it is able repair almost all of them but the sheer rising volume overwhelms it leaving some breaks unrepaired. However powerful this is just one stream of damage. As we age there a thousand different streams of damage. It is a wonder how we last as long as we do. Shows how resilient our biology is. Ginkgo Biloba tree has figured out a version of this resilience to stabilize its epigenome and nuclear architecture. I am surprised that why people are not astounded by this remarkable achievement by living multicellular species.
Well said… This is incredible and astounding.
Educational and inspiring! If possible, please add me to your regular distribution so I never miss your posts.
Thank you so much for your kind words Walter! I have to figure out why blogspot is not allowing follow requests.
Akshay, did you see the new paper Plasma membrane damage as a cause of cellular senescence? Maybe the stability of the cell is dependent on the membrane composition, rigidity and resistance to many types of damage, including the epigenome? What do you think?
https://www.oist.jp/news-center/news/2024/2/22/damage-cell-membranes-causes-cell-aging
Hi Leo, This is another symptom of aging and not the cause. Just like loss of NAD+. If the epigenetic marks we get from Nature’s template during embryogenesis and during differentiation remain where they should the membrane would continue to be healthy as any damage would be easily repaired. Since we are unable to maintain the epigenetic template currently one can look at incorporating grass fed Ghee in our diet. It is a good source of pentadecanoic acid and choline both of which strengthen cell membranes. Additionally Ghee is the highest food source of butyric acid and also contains conjugated linoleic acid both of which have health benefits.
Thank you Akshay. Everything is the symptom and every time we discover something new, we try to explain the mystery but…
Hopefully you will soon find out how to freeze it and before freezing, reversing at the homeostatic bliss.
I hope so too :)
Wonderful! Thank you.
Akshay what do you think about Mitochondria being the first and the most important thing causing the process and vicious cycle of aging?
Mitochondria can remain fit if the cell’s epigenetic signature remains intact and DNA/nuclear damage is minimal and repaired consistently.
Akshay, did you see the paper “Time is ticking faster for long genes in aging”
They say polymerase stalling following DNA damage is a cause of gene length dependent transcription decline.
I read an article mentioning Mitochondrial radicals as the cause of aging through damaging macromolecules.
Yes I loved that paper and it corroborates the paper I had cited in my blog post Agents of Time: ‘Aging is associated with a systemic length-associated transcriptome imbalance’ They come to similar conclusion that rampant double strand DNA breaks cause interruptions during transcriptions which affect longer transcripts more. So since all these changes are cascading it would affect multiple organelles deteriorating their function. This does not make them the primary cause of aging just like neither are senescent cells or lower NMN primary causes of aging. They are all collateral damage. Since mitochondria are the power sources of our cells and we are a collection of cells they are like distributed batteries that actually run us. So their malfunction is as serious if not more serious than the other symptoms of aging.
Thank you.
I am a little confused. I read your post and seems like repressed heat shock protein is behind aging. If we can upregulate it should stop the DNA breaks. However I’m one of your comments I see that you mention DNA breaks is just one of many damages. That’s why strengthening cell membrane may not be that effective.
in that case, what are all the other category of damages?
From your post I came away with the understanding that heat shock protein gets repressed during puberty. And that causes double dna breaks and that further damages long non coding rna transcription process.
I am not an expert on biological terms. So apologies if my question was redundant. But will appreciated if you can clarify.
Hi Bio_ehtusiast89, your question is not at all redundant. In fact it’s quite intelligent. The repression of heat shock proteins is given as an example of how changes made just after puberty launch a cascade of changes that slowly manifest into aging. For example neuronal loss in the hippocampus at age of 13. In my previous post Agents of Time I have mentioned about decline of Extracellular Matrix begins just after puberty. Cell = ECM communication is critical for cell survival and function. So there are many such deliberate changes occurring just after reproductive maturity that leads to thousands of consequences. But Gingko Biloba tree seems to either prevent these harmful changes from happening or they have developed the ability to reset the epigenetic landscape back to its template established at the time of differentiation from any perturbation. Planaria worms have the ability to undergo the same cleaning of the slate of errors process we humans undergo once during embryogenesis every time they are cut. May be we can learn to trigger this process every time we cross a threshold of errors. This will be the ultimate future of auto self regeneration keeping us young forever.
Akshay, what do you think of this latest paper in Nature: https://www.nature.com/articles/s43587-024-00612-4
Even though I am not a Biologist, this publication seems to be yet another validation of your work! They used the normal concentration of Extracellular Vesicles found in young mice. I wonder what would have happened if they had tried higher concentrations. According to your results, the magnitude of rejuvenation is highly depended on EV concentration.
Thank you for your comment. You have correctly inferred all your points. Yes this is a strong confirmation of our work. They also confirmed the role of regulatory ncRNAs which I have mentioned in my posts as the key to reset the epigenetic age of older cells to become younger.
Hi Akshay. Briefly said, aging is a loss of cellular and specifically, nuclear architecture, that the optimal and cell specific genes are no longer read.
Do you think Mitochondria is the real starter of this process through energy generation waste damaging cell components? And the second one should be telomere attrition since cells need to divide.
So we should fix those 2 for the first place, right? What would be other main and upstream causes of DNA damage and therefore, loss of architecture?
Hi Cancer cells show us that telomere length can indefinitely be extended by ensuring production of requisite telomerase. Mitochondria too seems to be affected due to aging rather than the other way round from published papers. One of the earliest age related change that has been found by Morrimoto and Lambada is drop in the efficiency of heat shock proteins, chaperones in protein production, by more than 60% just after puberty when mitochondria is working just fine. There are other such deliberate changes that start the cascade of age related deterioration. But what is wonderful is that all this damage is reversible.
But what causes the change in Heat Shock Protein production? Something should be starting this decline. Since mitochondria is responsible for the energy production for us to function, that’s why I thought it would be the main concern.
You are onto something :) here is another paper by Morimoto which says that a little stress on mitochondria actually restores the Heat Shock Respibse: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726777/
But to answer your question: Germline stem cells trigger this collapse probably to conserve all resources for puberty and reproduction. This is the Morimoto paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4546525/
Thank you for citing this paper. Do you know if restoring heat shock response and preserving Proteostasis collapse maintain youthful epigenome and homeostasis? Does increasing HSF-1 binding and RNA polymerase II recruitment at HSF-1 target genes and therefore, restoration of cytoplasmic proteostasis increase only vitality later in life or also extends lifespan dramatically?
Akshay, if collapse of proteostasis is
the earliest event in aging, why it’s considered as one of the downstream hallmarks?
Hi Well they probably missed the seminal paper by Morimoto and Lambada which shows how the heat shock protein chaperone support during protein production is deliberately diminished by 60%-70% just after puberty by epigenetic changes driven by germline stem cells. It is one a a few such significant changes that happen just after our biological peak at puberty. Another example is mentioned in my previous post ‘Agents of Time’ on decline extracellular matrix that occurs just after puberty https://karger.com/ger/article/66/3/266/148307/The-Matrisome-during-Aging-and-Longevity-A-Systems
Hi I missed the comment posted on 30th April. Proteostasis is obviously a very important process in running our biology just like healthy mitochondria. So if we are able to maintain nuclear architectural stability and thereby keep all such key players in our biology at optimal efficiency we can certainly hope for not only longer lifespan but longer youthful lifespan.
Post a Comment